SMM July 30 News:
The core differences and characteristics of garnet-type (LLZO), perovskite-type (LLTO), and NASICON-type (LATP) in oxide electrolyte systems, helping you clearly distinguish between these three types of materials:
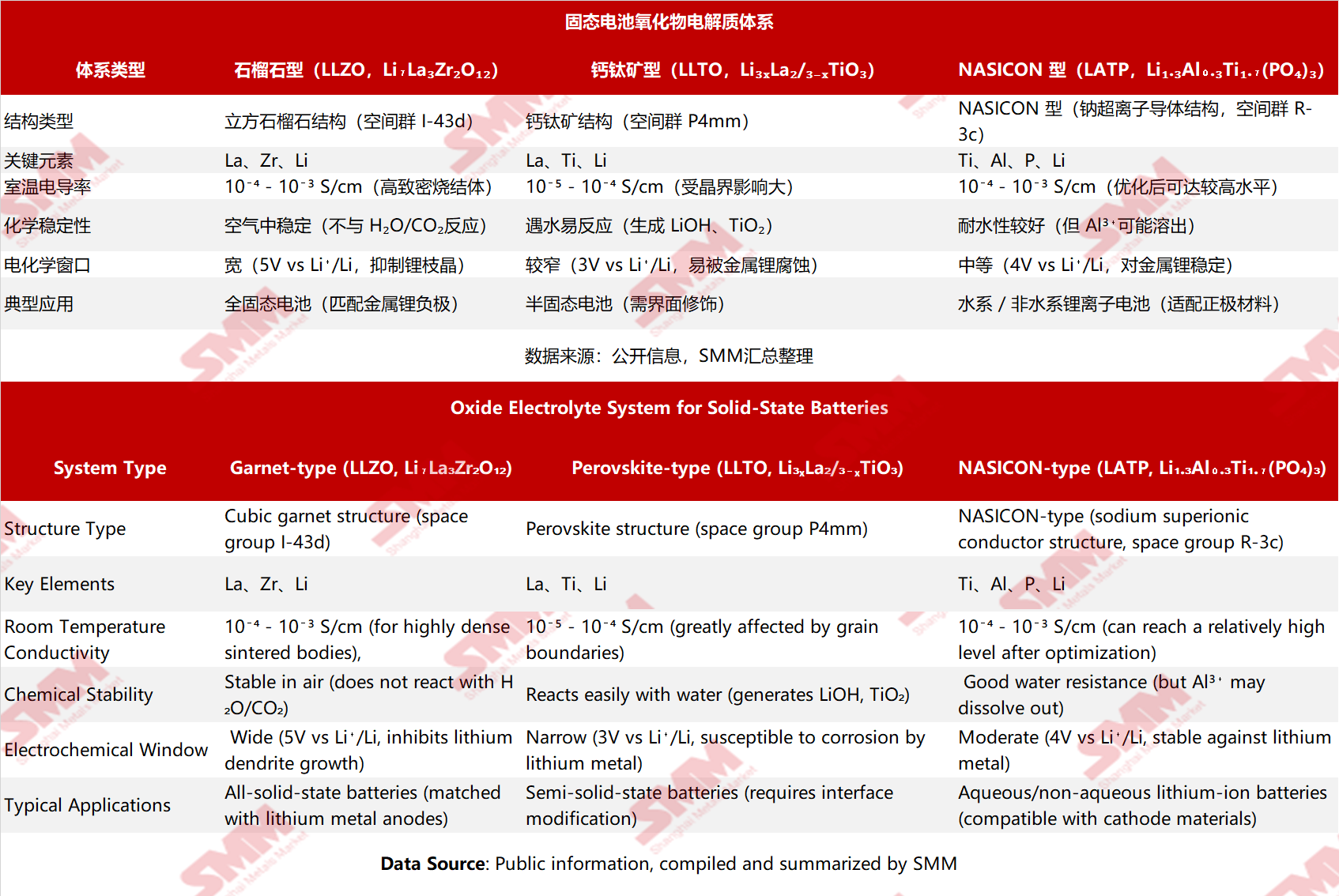
I. Comparison Table of Basic Information
II. In-depth Analysis of Core Differences
1. Structure and Ion Migration Mechanism
Garnet-type (LLZO):
In the cubic garnet structure, ZrO₆ octahedra and LaO₈ dodecahedra form a three-dimensional framework, with lithium ions jumping between tetrahedral/octahedral positions within the framework gaps. The migration path is continuous and "isotropic" (without significant directional differences), allowing for high ionic conductivity in highly dense sintered bodies (relative density > 95%).
Perovskite-type (LLTO):
In the perovskite structure (ABO₃ type), the A-site is occupied by Li/La, and the B-site by Ti. However, during actual synthesis, high grain boundary resistance often occurs (impeding lithium ion migration at grain boundaries), and lithium ion migration is influenced by the crystal's "anisotropy" (significant differences in conductivity in different directions), resulting in overall conductivity lower than the theoretical value.
NASICON-type (LATP):
In the NASICON structure (derived from Na₁+xZr₂P₃−xSiₓO₁₂), PO₄ tetrahedra and TiO₆ octahedra are connected at their vertices, forming three-dimensional ion channels. Lithium ion migration relies on an "ion exchange" mechanism (Li⁺ combines/dissociates with vacancies within the channels). Al³⁺ doping can expand the channel volume and increase vacancy concentration, thereby enhancing conductivity.
2. Chemical Stability and Interface Compatibility
LLZO: It can stably exist at the Li⁺/Li⁰ interface on its surface (with a wide electrochemical window up to 5V), directly matching lithium metal anodes (inhibiting lithium dendrite penetration), and does not react in humid air (eliminating the need for glovebox operations), making it an ideal candidate for all-solid-state batteries.
LLTO: It undergoes hydrolysis reactions when exposed to water (Li₃xLa₂/₃₋ₓTiO₃ + H₂O → LiOH + TiO₂ + La(OH)₃), leading to the formation of an insulating layer on the surface and significantly increasing interface resistance. Meanwhile, it is easily reduced when in contact with lithium metal (Ti⁴⁺ → Ti³⁺), requiring interface modification through methods such as Al₂O₃ coating.
LATP: It has better water resistance than LLTO (although long-term immersion may still lead to Al³⁺ dissolution), and is compatible with cathode materials (such as LiFePO₄). However, when in contact with lithium metal anodes, Li⁺ reduces P⁵⁺ (forming insulating phases such as Li₃P), making it unsuitable for lithium metal anodes and more suitable for pairing with "non-lithium metal" anodes such as graphite. 3. Preparation Challenges and Process Control
LLZO: It is necessary to address the issue of high-temperature sintering cracking (component segregation caused by Li volatilization). Typically, a two-step sintering method (first high-temperature synthesis followed by low-temperature densification) or the introduction of sintering aids (such as Li₂CO₃) is adopted. Meanwhile, the Zr/La ratio needs to be controlled (excessive Zr can stabilize the cubic phase).
LLTO: It is prone to forming impurity phases (such as La₂Ti₂O₇), so the synthesis temperature (~1000℃) and atmosphere (inert atmosphere to prevent Ti reduction) need to be strictly controlled. Additionally, due to its high grain boundary resistance, it is necessary to reduce the resistance through nanocrystallization (such as preparing nanoparticles via the sol-gel method) or grain boundary modification (adding Li₃BO₃).
LATP: The key lies in the uniformity of Al³⁺ doping (which affects the concentration of channel vacancies). The sol-gel method can achieve atomic-level doping, but it is costly. The traditional solid-phase method requires precise control of the sintering temperature (~900℃) and time to avoid TiO₂ phase separation.
4. Application Scenarios and Technical Bottlenecks
LLZO: It is suitable for all-solid-state lithium metal batteries (such as the R&D direction of Toyota and CATL). The bottlenecks are the high sintering cost (requiring high-temperature densification and high energy consumption) and the interfacial resistance with the cathode (requiring coating modification, such as LiNbO₃).
LLTO: Due to its poor interfacial stability, it is more suitable for semi-solid-state batteries (combined with liquid electrolytes to reduce interfacial resistance). However, its poor compatibility with lithium metal anodes limits its application in high-energy-density batteries.
LATP: It has been pilot-applied in aqueous lithium-ion batteries (such as ESS batteries) (utilizing its water resistance). However, due to its compatibility issues with lithium metal anodes, it is difficult for it to enter the high-energy-density solid-state battery market. It is more often used as an "auxiliary electrolyte" (such as being combined with polymers).
III. Summary: How to Choose Among the Three Types of Oxide Electrolytes?
For high safety + lithium metal anode → choose LLZO (chemically stable, inhibits lithium dendrite growth, suitable for all-solid-state batteries).
For low-cost semi-solid-state battery pilot applications → choose LLTO (raw materials are inexpensive, but interfacial issues need to be addressed).
For aqueous/non-lithium anode batteries → choose LATP (good water resistance, compatible with traditional cathodes). IV. Enterprise Layout in Oxide Electrolyte Batteries
Ganfeng Lithium: Possesses a diversified technical route for solid-state batteries, covering oxides, sulphides, polymers, etc. The room-temperature ionic conductivity of its oxide solid electrolytes LLZO and LATP can reach 1.7 mS/cm and 1.4 mS/cm, respectively. The developed 5-micron ultra-thin oxide electrolyte membrane effectively reduces interfacial impedance by up to 40%. The 5 GWh hybrid solid-liquid battery production line at its Chongqing base has commenced operation, with the energy density of all-solid-state batteries exceeding 500 Wh/kg. It plans to mass-produce all-solid-state batteries in 2025, which will be paired with car models such as Dongfeng VOYAH.
Great Power Energy: Completed the development of the first-generation oxide all-solid-state battery product in March 2025, with an energy density target exceeding 300 Wh/kg. It adopts a sandwich structure scheme to improve interfacial contact issues and expects to establish a production line and commence mass production in 2026. The cost of its oxide solid-state battery is only 15% higher than that of liquid batteries. Its mass production line in Changzhou has been established, with a discharge retention rate of 92% at -20℃, suitable for car models such as Wuling Binguo.
Shanghai Xiba
The only enterprise in China to achieve ton-scale mass production of LLZO oxide electrolytes, with a yield rate as high as 98%. In 2025, its capacity will be expanded to 2,000 mt/year, supporting BYD's blade solid-state battery project, with costs 40% lower than those of the sulphide route. Its multi-form solid-state electrolyte powder materials adopt an oxide technical route, and some products have already been applied in the consumer battery field.
Narada Power
Released oxide energy storage solid-state batteries with an ionic conductivity as high as 10⁻³ S/cm in April 2025. Specific parameters such as the energy density of its 783 Ah ultra-large capacity energy storage solid-state battery have not yet been clarified.
BTR: Commenced ton-scale shipments of oxide solid-state electrolyte products in 2024, with a total room-temperature ionic conductivity exceeding 5×10⁻⁴ mS/cm.
Jinlongyu: Announced in April 2025 its intention to invest in and construct a mass production line project for key materials of solid-state batteries in Huizhou, with a construction period expected to be 12 months and no more than three years. Its solid-state battery technology centers on an oxide electrolyte system.
China Automotive Innovation & Intelligence: Has acquired the kg-scale preparation capability for electrolytes, with a room-temperature ionic conductivity reaching 0.7-1.0 mS/cm. Duer Automotive Parts: Focusing on the oxide electrolyte route, while also maintaining technical reserves in polymer and sulphide technologies. Construction of the pilot production line in Huzhou will commence in June 2025, with an investment of 300 million yuan. It is planned to be completed by the end of the year and have preliminary capacity, followed by plans for a 1GWh mass production line. Passed third-party safety testing in Japan in 2023, and will showcase the second-generation product in 2025, with an energy density of 260Wh/kg. The third-generation product aims for 400Wh/kg.
Qingtao Energy: Adopting a composite electrolyte of oxide + polymer to improve interfacial contact issues, with plans to launch all-solid-state batteries in 2028. The semi-solid-state battery has an energy density of 350-400Wh/kg and has been installed in the NIO ET7. The Taizhou base will commence production in 2025, with a capacity of 10GWh.
CALB: Launched the "Boundary-less" all-solid-state battery in August 2024, with an energy density of 430Wh/kg and a capacity exceeding 50Ah. It adopts the oxide technology route and will undergo small-batch vehicle installation verification in 2027.
WELION New Energy: Plans to achieve mass production of semi-solid-state batteries in 2026, with safety verified through nail penetration tests. It adopts the oxide + polymer technology route and plans to achieve mass production of all-solid-state batteries in 2027.
ProLogium Technology: Increased solid-state energy density to 350-390Wh/kg in 2024. After 2025, it will gradually replace the anode and cathode with lithium-rich manganese-based materials and lithium metal/anode-free alternatives, achieving a maximum energy density of 480Wh/kg. It adopts the composite route of oxide + polymer.
SVOLT Energy Technology: Has developed the first and second-generation jelly batteries. In July 2024, it released a patent for a composite solid-state electrolyte of oxide + polymer, adopting the oxide + polymer technology route.
GSP Automotive Group:
Will continue to advance R&D in solid-state and semi-solid-state batteries in 2026. The Wenzhou factory is expected to reach full production by mid-2026 and commence partial production by the end of the year, adopting the oxide + polymer technology route.
Tailan New Energy: Developed the world's first vehicle-grade 120Ah composite solid-state battery of oxide + polymer, with an energy density of 720Wh/kg. It is expected to complete prototype verification and system development in 2025, undergo continuous verification through small-batch production in 2026, and achieve mass production and demonstration applications in NEVs in 2027. Gotion High-tech: The semi-solid battery with oxide + polymer has an energy density of 360Wh/kg, and the corresponding car model achieves a driving range exceeding 1000km. Loading verification will commence in 2025.
Oxide solid-state batteries exhibit strong electrochemical stability, high mechanical strength, easy electrode matching, and good environmental stability. However, their ionic conductivity is relatively low, and the interfacial impedance needs further reduction. In contrast, sulphide solid-state batteries have high ionic conductivity, good interfacial contact performance with electrode materials, and high theoretical energy density. However, they are sensitive to water and oxygen, require stringent preparation and storage conditions, and have higher costs.
The advancement speed of solid-state batteries following the oxide route may lag behind that of sulphide route solid-state batteries, which have higher conductivity and energy density.
**Note:** For further details or inquiries regarding solid-state battery development, please contact:
Phone: 021-20707860 (or WeChat: 13585549799)
Contact: Chaoxing Yang. Thank you!



