SMM1 11: over the years, China's economy has maintained a high growth rate, which provides a good basis for the development of China's galvanizing industry. China has become a large production and consumption country of hot-dip galvanized steel. Recently, China's economic development has entered a period of steady growth, and the galvanizing industry has entered a new period of development.

Galvanizing refers to the surface treatment technology of plating a layer of zinc on the surface of metal, alloy or other materials to play the role of beauty, rust prevention and so on. The main method is hot galvanizing. Relevant scholars and experts put forward that hot galvanizing is a traditional industry, in the current situation of overcapacity and increasing environmental protection, it is necessary to increase technological innovation, actively promote common technology, and use Internet technology to promote the construction of intelligent factories. Research and development and promotion of waste gas, waste liquid, waste residue collection and treatment technology, energy saving and emission reduction technology, corrosion-resistant materials and alloys. Speed up the formulation of industry standards and standardize the market with standards. To create a good environment for the sustainable development of hot-dip galvanizing industry.
Zinc is soluble in acid and alkali, so it is called an bisexual metal and hardly changes in dry air. In wet air, a dense basic zinc carbonate film is formed on the surface of zinc. In the atmosphere containing sulfur dioxide, hydrogen sulfide and marine atmosphere, the corrosion resistance of zinc is poor, especially in the atmosphere of high temperature and humidity containing organic acid. The standard electrode potential of zinc is-0.76V. For iron and steel substrate, zinc coating belongs to anodic coating, which is mainly used to prevent the corrosion of iron and steel, and its protective performance is closely related to the thickness of the coating. After passivation treatment, dyeing or coating with light protector, zinc coating can significantly improve its protection and decoration.
What are the categories?
Cold galvanizing
Cold galvanizing, also known as galvanizing, is the use of electrolytic equipment to remove oil, pickle, and then put the pipe into a solution composed of zinc salt, and connect the negative pole of the electrolytic equipment, place a zinc plate on the opposite side of the pipe fitting, and connect to the positive pole of the electrolytic equipment to turn on the power supply. Using the directional movement of the current from the positive pole to the negative pole, a layer of zinc will be deposited on the pipe fittings. The cold-plated pipe fittings are processed first and then galvanized.

Mechanical galvanizing
In a rotating rolling barrel containing a plated piece, a glass ball, a zinc powder, water and an accelerator, the glass ball as an impact medium rotates with the barrel, and friction and hammering occurs with the surface of the plated piece to produce mechanical and physical energy, under the action of a chemical accelerator, The coated zinc powder is "cold welded" to the surface of the coating to form a smooth, uniform and meticulous coating with a certain thickness.
The technical standard adopts American ASTM B695 2000 and military C 81562 mechanical galvanizing technical standard.
Hot dip galvanizing
At present, the main method of galvanizing on the surface of steel plate is hot galvanizing.

Hot-dip galvanizing is developed from the older hot-dip galvanizing method. It has a history of nearly 200 years since France applied hot-dip galvanizing to industry in 1836. However, the hot-dip galvanizing industry has been developed on a large scale with the rapid development of cold rolled strip in the past three decades.
The production process of hot-dip galvanized sheet mainly includes the inspection of finished products after hot-dip plating and so on. According to the custom, according to the different pretreatment methods, the hot dip galvanizing process is divided into two categories: out-of-line annealing and in-line annealing. That is, wet method (single sheet steel hot-dip galvanizing method), out-of-line annealing (single sheet steel hot-dip galvanizing method), hot-dip galvanizing Huilin (Wheeling) method (strip continuous hot-dip galvanizing method), in-line annealing Senjimir (Sendzimir) method (protective gas method), Modified Senjimir method, American steel association method (same as Kawasaki method, Japan), Silas (Selas) method and Sharon (Sharon) method.
Out-of-line annealing
Before hot rolled or cold rolled steel plate enters the hot galvanizing line, the hot rolled or cold rolled steel plate is first annealed in the bottom annealing furnace or bell annealing furnace, so that there is no annealing process in the galvanized line. Steel plates must maintain a clean, pure iron active surface free of oxides and other dirt before hot-dip galvanizing. In this method, the annealed iron oxide scale is removed by pickling, and then protected by zinc chloride or a mixture of ammonium chloride and zinc chloride, so as to prevent the steel plate from being oxidized again.
(1) Wet hot-dip galvanizing
The solvent on the surface of the steel plate enters the zinc solution covered with molten solvent without drying (that is, the surface is still wet) for hot galvanizing. The disadvantages of this approach are:
a. It can only be galvanized in a lead-free state, and the alloy layer of the coating is very thick and the adhesion is very bad.
b. The resulting zinc slag is accumulated at the interface between liquid zinc and lead liquid and can not be deposited at the bottom of the pot (because the specific gravity of zinc slag is larger than that of zinc liquid and less than that of lead liquid), so that the steel plate pollutes the surface by passing through the zinc layer. Therefore, this method has been basically eliminated.
(2) single steel plate
In this method, the hot rolled plate is generally used as the raw material, and the annealed steel plate is first sent to the pickling workshop to remove the iron oxide scale on the surface of the steel plate with sulfuric acid or hydrochloric acid. After pickling, the steel plate immediately enters the water tank and waits for galvanization, which can prevent the steel plate from reoxidation. After pickling, water cleaning, extrusion drying, into the zinc pot (the temperature has been maintained at 445-465 ℃) hot-dip galvanizing, and then oil and chromium treatment. The quality of hot-dip galvanized sheet produced by this method is significantly higher than that of wet galvanized products, and only has a certain value for small-scale production.
(3) Huilin fever
The continuous galvanizing production line includes a series of pretreatment processes, such as alkali debinding, pickling, water washing, solvent coating, drying and so on, and the bell furnace needs to be carried out before the original plate enters the galvanizing line. The production process of this annealing method is complex and the production cost is high. What is more important is that the products produced by this method often have solvent defects, which affect the corrosion resistance of the coating. In addition, the AL in the zinc pot often interacts with the solvent on the surface of the steel plate to form aluminum trichloride, and the adhesion of the coating becomes worse. Therefore, although this method has been published for nearly 30 years, it has not been developed in the world hot-dip galvanizing industry.
In-line annealing
That is, the strip coil is directly provided by the cold rolling or hot rolling workshop as the original plate of hot-dip galvanizing, and the hot-dip galvanizing line is annealed by gas-protected recrystallization in the hot-dip galvanizing line. Hot-dip galvanizing methods belonging to this industry include: Senjimir method, modified Sinjimir method, American steel joint method (same as Kawasaki method, Japan), Silas method, Sharon method.
(1) Senjimir method
It is a combination of annealing process and hot-dip galvanizing process, and its in-line annealing mainly consists of oxidation furnace and reduction furnace. The strip is directly heated to about 450 degrees by gas flame in the oxidation furnace, and the remaining rolling oil on the surface of the strip is burned off to purify the surface. After that, the strip is heated to 700 to 800 degrees to complete the recrystallizing annealing, and the temperature before entering the zinc pot is controlled at about 480 degrees by the cooling section, and finally enters the zinc pot without touching the air. Therefore, the Sengimir method has high output and good galvanizing quality. This method has been widely used.
(2) American steel joint method
It is a variant of the Senjimir process, it only uses an alkaline electrolytic degreasing cell to replace the degreasing effect of the oxidation furnace, and the other processes are basically the same as the Senjimir process. After the original plate enters the operation line, the electrolytic defatting is carried out first, then washed and dried, and then recrystallized and annealed through a reduction furnace with protective gases, and finally hot-dip galvanized into a zinc pot under the condition of sealing. Because the strip is not heated by the oxidation furnace, the oxide film on the surface of the strip is thin, and the hydrogen content of the protective gas in the reduction furnace can be reduced properly. In this way, it is beneficial to the safety of the furnace and the reduction of production cost. However, because the strip is not pre-heated into the reduction furnace, which undoubtedly increases the heat load of the reduction furnace and affects the life of the furnace. Therefore, this method has not been widely used.
(3) Silas method
Also known as flame direct heating method; First, the strip is defatted by alkali washing, then the oxide scale on the surface is removed by hydrochloric acid, and after washing and drying, it enters the vertical line annealing furnace directly heated by gas flame, by strictly controlling the flame burning ratio of gas and air in the furnace. Make it in the case of excess gas and lack of oxygen to carry out incomplete flame burning, so as to create a reduction atmosphere in the furnace. The strip is rapidly heated to the recrystallizing temperature and cooled in low hydrogen protective atmosphere. Finally, the strip is immersed in zinc solution under airtight condition for hot galvanizing. The method has the advantages of compact equipment, low investment cost and high output (up to 50 / hour). However, the production process is complex, especially when the unit stops running, in order to avoid burning off the strip, it is necessary to use the furnace to move away from the steel strip, so there are many problems in this operation, so this method is rarely used in hot-dip galvanizing industry.
(4) Sharon method
In 1939, Sharon Company of the United States put into production a new type of hot-dip galvanizing unit, so it is also called Sharon method. This method is to spray hydrogen chloride gas into the strip steel in the annealing furnace and make the strip reach the recrystallization temperature, so it is also called gas pickling method. By using hydrogen chloride gas pickling, not only the oxide scale on the surface of the strip can be removed, but also the grease on the surface of the strip can be removed at the same time. Because the surface of the strip is corroded by oxidation gas, the hemp surface is formed. Therefore, the adhesion of the coating obtained by Sharon method is particularly good. However, due to the serious corrosion of equipment, resulting in a very high cost of equipment maintenance and renewal. Therefore, this method is rarely used.
(5) improved Jimmy Mori
It is a superior hot-dip galvanizing process. It connects the independent oxidation furnace and the reduction furnace in the Senjimir method by a small aisle, so that the whole annealing furnace, including the preheating furnace, the reduction furnace and the cooling section, forms an organic whole. Practice has proved that this method has many advantages: high quality, high yield, low consumption, safety and so on. Its development speed is very fast, almost all the new operation lines have adopted this method since 1965, and most of the old Senjimir units have been reformed according to this method in recent years.
Principle
The hot dip zinc layer is formed by zinc in liquid state in three steps. The iron base surface is dissolved by zinc liquid to form zinc and ferroalloy phase layer; the zinc ion in the alloy layer further diffuses to the matrix to form zinc and iron intersoluble layer; the alloy layer is coated with zinc layer.
Performance characteristics
The thick and dense pure zinc layer covers the surface of iron and steel fasteners, which can avoid the contact between steel matrix and any corrosion solution and protect the matrix of iron and steel fasteners from corrosion. In the general atmosphere, the surface of zinc layer forms a very thin and dense surface of zinc oxide layer. It is difficult to dissolve in water, so it plays a certain protective role in the matrix of iron and steel fasteners. If zinc oxide and other components in the atmosphere form insoluble zinc salt, the anti-corrosion effect is more ideal, and it has zinc-ferroalloy layer, which is compact and shows unique corrosion resistance in marine salt fog atmosphere and industrial atmosphere, and its corrosion resistance is better if zinc oxide and other components in the atmosphere form insoluble zinc salt. Because of the strong bonding, zinc-iron mutual solubility, has a strong wear resistance; Because zinc has good extensibility and its alloy layer is firmly attached to the iron and steel substrate, hot-dip galvanizing can carry out various forming processes, such as cold stamping, rolling, wire drawing, bending and so on, without damaging the coating. After hot-dip galvanizing, it is equivalent to one-time annealing treatment, which can effectively improve the mechanical properties of steel matrix, eliminate the stress during forming and welding, and is beneficial to the turning of steel structure parts. the surface of the fastener after hot galvanizing is bright and beautiful. Pure zinc coating is the most plastic zinc coating in hot dipping zinc, and its properties are similar to those of pure zinc and have good extensibility.
Application range
This plating method is especially suitable for all kinds of strong acid, alkali fog and other strong corrosion environment.
Electroplating zinc
1. Principle
Because zinc is not easy to change in dry air, and in wet air, the surface can form a very dense zinc carbonate film, which can effectively protect the interior from corrosion. And when the coating is damaged and not too large matrix is exposed for some reason, zinc and steel matrix form a microbattery, which makes the fastener matrix become the cathode and be protected. It is widely used in automobile transportation and other industries, but what is needed is trivalent chromium passivation layer and zinc-nickel alloy plating closed coating to reduce the harmful and toxic layer of hexavalent chromium passivation.
2. Performance characteristics
Zinc coating is thick, fine crystallization, uniform and no pores, good corrosion resistance; The zinc layer obtained by electroplating is relatively pure, corrodes slowly in acid, alkali and other fog, and can effectively protect the fastener substrate. After passivated by chromic acid, the galvanized layer forms white, color, military green, etc., beautiful and generous, with a certain degree of decoration. Because the galvanized coating has good extensibility, it can be formed by cold punching, rolling, bending and so on without damaging the coating.
3. Scope of application
The field of electroplating zinc is more and more extensive, the application of fastener products has spread throughout the mechanical manufacture, the manufacture of galvanized hook flower net, electronics, precision instruments, chemical industry, transportation, aerospace and so on are of great significance in the national economy.

What are the processes?
There are two kinds of galvanizing processes: hot galvanizing and cold galvanizing. Cold galvanizing is also known as galvanizing. Zinc plating is mainly discussed here.
There are many kinds of electroplating zinc. However, from the PH value of zinc plating bath, there are two main categories: alkaline zinc plating and acid zinc plating.
Alkaline zinc plating
Alkaline zinc plating process refers to the PH value of the bath is alkaline. However, due to the different complexing agents, it can be divided into two types: (CN) 2 zinc plating and zincate zinc plating. (CN) 2 galvanizing is a very old plating seed. There are three main components in the bath: main salt zinc oxide, complexing agent (CN) 2 sodium hydroxide and conductive salt sodium hydroxide (commonly known as fire alkali). There was no brightener in the early (CN) 2 plating solution. With the improvement of people's aesthetic requirements, a brightener was added to the zinc plating solution of (CN) 2 chemical bath. The process of (CN) 2 galvanizing is stable and the coating is meticulous. The dispersion ability of the plating bath is good. According to the content of sodium (CN) 2, it can be divided into three types: high (CN) 2, medium (CN) 2 and low (CN) 2 galvanizing. The biggest disadvantage of (CN) 2 galvanizing is that it is too toxic and harmful to the environment.
Zincate zinc plating is a rapidly developing zinc plating process in the past 30 years. Its main composition is the main salt zinc oxide, complexing agent and conductive salt sodium hydroxide (commonly known as fire alkali). In order to obtain fine dispersion ability of the bright coating, but also add brightener. The main development period of domestic zincate galvanizing is the (CN) 2 galvanizing era in the 1970s. The famous DPE galvanizing process and DE galvanizing process have been used until now. Although this process is not as stable and meticulous as the (CN) 2 galvanizing process. But its biggest advantage is that it has no (CN) 2. The harm to the environment is much less. Now zincate zinc plating has new development, blistering and brittleness and other defects have been overcome, dispersion ability has been greatly improved, and can be compared with (CN) 2 galvanized zinc plating.
Acid galvanizing
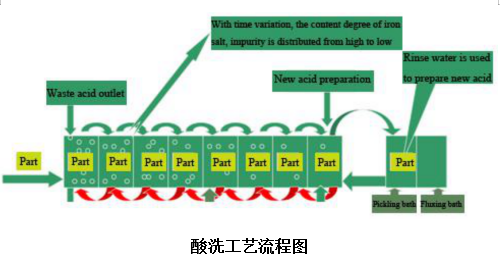
The PH value of the bath is between 4 and 6. The second achievement of (CN) 2 galvanizing in the 1970s was (CN) 2 ammonium chloride galvanizing. On a par with zincate alkaline galvanizing. It takes zinc chloride as the main salt, ammonium chloride as the complexing agent and conductive salt. Citric acid and NH3 triacetic acid were used as auxiliary complexing agents. Polyethylene glycol and thiourea are used as coating refiners. The coating obtained from this bath is fine and has good dispersion ability. The toxicity of the bath is small. However, the main disadvantage is that the plating solution is unstable and the ammonium chloride gas emitted is very corrosive to the electroplating equipment. The current efficiency is also low. It is sensitive to impurities. The working temperature range is narrow. It has been basically eliminated by the potassium chloride zinc plating process.
Zinc plating process of potassium Chloride (or Sodium Chloride)
Potassium chloride (or sodium chloride) galvanizing process is a new (CN)-2 free galvanizing process developed in 1980s. Its main composition is: zinc chloride main salt, the general content is 70-90g/L. Potassium chloride is used as conductive salt, the content of potassium chloride varies from 140-280g/L, and can be adjusted arbitrarily according to different needs. Boric acid was used as PH buffer to stabilize PH between 4.6 and 5.6. Because the bath works at room temperature, the solubility of boric acid is not high, and the content of boric acid is generally controlled in 25-30g/L.
These three components alone can not produce qualified zinc coating. Some additives need to be added to obtain a bright and meticulous zinc coating. The advantages of potassium chloride zinc plating are stable bath, bright and meticulous coating, low cost, high current efficiency and non-toxic. The disadvantage is that the dispersion ability of the bath is slightly worse than that of alkaline zinc plating, and the brittleness of the coating is also larger. Nevertheless, potassium chloride galvanizing came out and was immediately and generally popular. The development is very rapid, has occupied half of the galvanized rivers and mountains. From the market point of view, the current galvanizing process is mainly two kinds of zinc plating: alkaline zincate and acid potassium chloride zinc plating. Other galvanizing processes have been dwarfed by the slow withdrawal from the historical stage.
There is also a sulfate galvanizing process in the acid galvanizing process. Its main component is the main salt zinc sulfate. The content is between 250-500g/L. Alum or aluminum sulfate as conductive salt. The content is between 30-50g/L. Conductive salt also adds sodium sulfate or sodium chloride. In addition to the above several, but also add some additives, the function is to make the coating meticulous. The first is the use of dextrin or gum, and later invented a number of special brighteners to make the coating brighter and more detailed. The biggest advantage of sulfate zinc plating is that it can use high current density (1-5A/dm2) and fast plating speed. However, the disadvantage is that the dispersion ability of the plating solution is very poor, so it is not suitable to be used for plating more complex workpieces.

Scan QR code, apply to join SMM metal exchange group, please indicate company + name + main business



