SMM April 24 News:
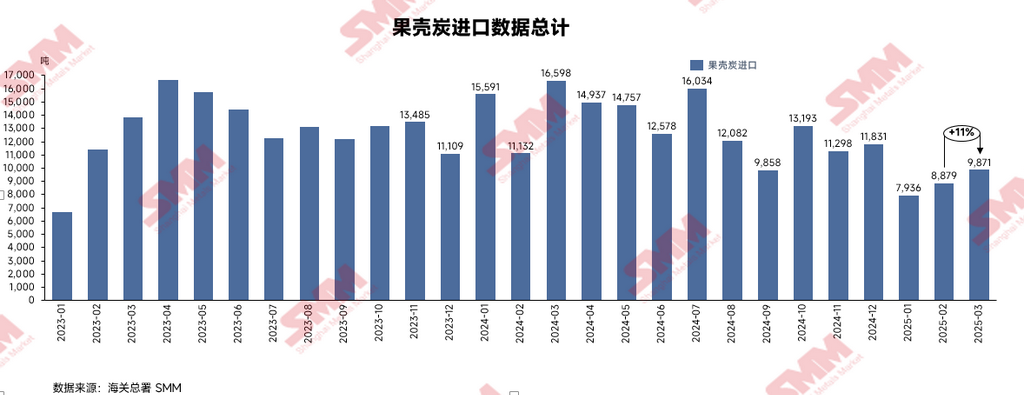
According to the latest data from the General Administration of Customs, the import volume of nutshell carbon in March 2025 was 9,871.1 mt, up 11% MoM but down 40.5% YoY. The average import price of nutshell carbon in March was $492.39/mt.
Imported nutshell carbon has a wide range of domestic applications, including industrial adsorption and purification, water purification, soil improvement, and use as a hard carbon anode in sodium-ion battery cells. Coconut shells, as agricultural waste, with their natural porous structure and high carbon content (about 50%), provide an ideal precursor for hard carbon production. Compared to traditional raw materials such as petroleum coke, coconut shell-based hard carbon offers significant advantages such as low cost, sustainability (global annual production of coconut shells exceeds 20 million mt), and environmental friendliness. So how does coconut shell carbon transform into a hard carbon anode?
Step-1: Modification of Coconut Shell Carbon
Pretreatment Stage
After crushing and sieving coconut shells to 20-40 mesh, the "acid-base two-step method" is used to remove impurities: first, soaking in 5% NaOH solution at 80°C for 12 hours to remove ash, then acid washing with 3% HCl solution to remove metal ions, and finally obtaining coconut shell powder with a moisture content of < 2% through flash drying technology. This process reduces the ash content from an initial 3.5% to below 0.3%.
Activation and Pore Formation Process
The KOH activation method is used to construct a hierarchical pore structure: coconut shell powder is mixed with KOH in a 1:3 ratio, heated to 800°C at a rate of 5°C/min in a nitrogen atmosphere, and held for 2 hours. During this process, KOH reacts with carbon (6KOH + 2C → 2K + 3H₂↑ + 2K₂CO₃), and the generated CO₂ gas etches the carbon skeleton, forming a mesoporous structure with a specific surface area of 1,200-1,500 m²/g.
High-Temperature Carbonization and Stabilization
The activated product undergoes secondary carbonization in an inert atmosphere at 1,200-1,400°C, forming a stable hard carbon structure by controlling the heating rate (10°C/min) and holding time (4 hours). In this stage, the graphitization degree (La) increases from an initial 2.1 nm to 3.5 nm, and the interlayer spacing (d002) stabilizes at 0.37-0.39 nm, meeting the requirements for sodium ion intercalation.
Surface Engineering Optimization
The "carboxyl-carbonyl bifunctional coating technology" is used to improve interface performance: hard carbon powder is ultrasonically dispersed with citric acid (mass ratio 1:0.1) in ethanol solution for 2 hours, vacuum-dried (120°C, 12 hours), and then heat-treated in an argon atmosphere at 400°C for 2 hours. XPS analysis shows that the coating layer introduces 0.8 at% of C=O bonds, reducing the SEI film impedance from 320 Ω to 120 Ω.
Step-2: Electrode Preparation and Performance Testing
Modified hard carbon (80%), Super P (10%), and PVDF (10%) are stirred into a slurry in NMP, coated onto copper foil (areal density 1.5 mg/cm²), and vacuum-dried at 80°C for 12 hours to form the electrode. In half-cell testing, this hard carbon anode exhibits a reversible capacity of 280 mAh/g, with the initial Coulombic efficiency increasing to 85%, and a capacity retention rate of 92% after 200 cycles. When matched with a sodium iron phosphate cathode, the full-cell energy density reaches 105 Wh/kg, with a cycle life exceeding 1,500 cycles.
Step-3: Key Technological Breakthroughs for Industrialization
Continuous Production Equipment: A microwave-assisted carbonization furnace was developed, reducing the production cycle from 24 hours in traditional processes to 6 hours, with a 40% reduction in energy consumption.
Intelligent Modification System: Based on machine learning algorithms, precise control of the activator ratio (KOH/C) and carbonization temperature is achieved, improving product batch stability to 98%.
Low-Cost Electrolyte: A mixed system of ethylene carbonate (EC)/dimethyl carbonate (DMC)/ethyl methyl carbonate (EMC)=3:3:4 is used, combined with 1.2 M NaClO4 salt, reducing costs by 60% compared to traditional lithium salts.
Currently, coconut shell-based hard carbon still faces challenges such as low tap density (0.6-0.8 g/cm³) and insufficient high-rate performance (10C capacity retention rate < 60%). Future breakthroughs in performance bottlenecks may be achieved through nanostructure design (e.g., preparing hard carbon/graphene composites) and electrolyte optimization (e.g., using ionic liquid electrolytes). With the surge in global demand for renewable energy storage, coconut shell-based hard carbon anodes are expected to achieve large-scale commercial application by 2030, driving the cost of sodium-ion batteries below ¥0.3/Wh.

SMM New Energy Research Team
Cong Wang 021-51666838
Rui Ma 021-51595780
Disheng Feng 021-51666714
Yanlin Lyu 021-20707875



